Confining the Sol-Gel Reaction at the Water/Oil Interface: Creating Compartmentalized Enzymatic Nano-Organelles for Artificial Cells
Living organisms compartmentalize their catalytic reactions in membranes for increased efficiency and selectivity. Here we developed a mild approach for in situ encapsulating enzymes in aqueous-core silica nanoreactors that can mimic the basic structure and reactions of organelles in the eukaryotic cells.

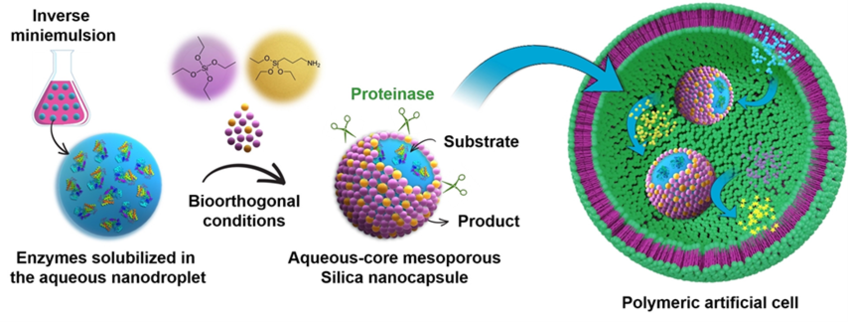
Compartmentalization is an essential characteristic of life because it enables spatiotemporal control over biological processes in the living cells. Cellular compartments, such as organelles and biomolecular condensates, provide a confined space that increases the efficiency and selectivity for enzymatic reactions and separates biological processes from the environment. To mimic the organelles of eukaryotic cells, we developed a mild approach for in situ encapsulating enzymes in aqueous-core silica nanocapsules. In order to confine the sol-gel reaction at water/oil interface of miniemulsion, we introduced an aminosilane to the silica precursors, which serves as both catalyst and an amphiphilic anchor that electrostatically assembles with negatively charged hydrolyzed alkoxysilanes at the interface. The semi-permeable shell protects enzymes from proteolytic attack, and allows the transport of reactants and products. The enzyme-carrying nanocapsules, as synthetic nano-organelles, are able to perform cascade reactions when enveloped in a polymer vesicle, mimicking the hierarchically compartmentalized reactions in eukaryotic cells. This in situ encapsulation approach provides a versatile platform for the delivery of biomacromolecules in the applications of disease treatment and synthetic biology.