Dipeptide coacervates as artificial membraneless organelles for bioorthogonal catalysis
Artificial organelles made of dipeptide coacervates enable bioorthogonal catalysis inside cells. These coacervates provide a stable, biocompatible, and hydrophobic microenvironment that effectively encapsulates and enhances the efficiency of transition metal-based catalysts in aqueous environments. When incorporated into cells, they function as active organelles that facilitate specific non-biological internal chemical reactions.
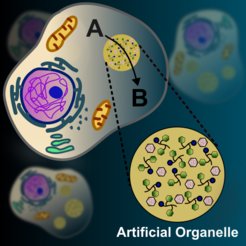
Eukaryotic cells achieve precision and efficiency in complex biological reactions by organizing them into compartments called organelles. These intracellular organelles create optimized environments that enhance, protect, and localize cellular processes and biological catalysts. Engineered materials that mimic cellular compartmentalization can be used to create synthetic systems with bioinspired properties.
A paper in Nature Communications presents the design of artificial organelles and microreactors with bioorthogonal catalytic activity. These systems were created using coacervates made from a diphenylalanine peptide derivative that form micron-sized liquid droplets under mildly basic conditions. The droplets concentrate hydrophilic enzymes and hydrophobic catalysts and act as reaction hubs in aqueous environments. Catalysts inactive in water were incorporated into these coacervates to create microreactors with bioorthogonal and photocatalytic activity in aqueous solutions. To demonstrate their biotechnological potential, dipeptide coacervates with a ruthenium catalyst were incorporated into living cells. These artificial organelles allowed the intracellular production of an active fluorophore mediated by the ruthenium complex.
This innovation in artificial organelles paves the way for reprogramming cellular chemistry and introducing non-biological pathways into cells, with promising implications for cell-targeted therapies, localized diagnostics, and synthetic biology.












