DFG: "Dissecting the nuclear pore‐like permeability barrier function of phase separated liquid FG nucleoporin condensates"
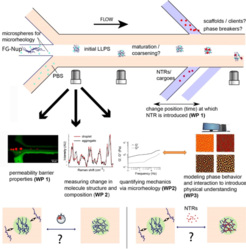
The nuclear pore complex (NPC) is a ~120 MDa complex built from multiple copies of ~30 different nucleoporins (Nups). The NPC traverses the nuclear envelope and functions as “gatekeeper” for nucleocytoplasmic transport. The NPC’s permeability barrier consists of ~10 different “FG-Nups”, primarily disordered Nups containing domains rich in phenylalanine (F) and glycine (G). These FG-domains non-covalently interact to form a supramolecular matrix of elusive structure in the NPC’s central region. A promising approach to study the permeability barrier is to develop in vitro reconstitution of FG-Nups into models that capture key features of the functional NPC, i.e. cargoes bigger then ~ 4nm cannot penetrate the barrier, unless they are bound to nuclear transport receptors (NTRs).
To investigate how this liquid FG-Nup permeability barrier functions, we now aim to build a unique “multi-analytic” platform that integrates microfluidics with coherent anti-Stokes Raman spectroscopy (CARS) and particle tracking microrheology (PTM), to study the evolving supramolecular structure as well as the mechanical properties of FG-rich droplets. The microfluidic device allows for controlled addition of NTRs into the FG-Nup solution at any stage during LLPS. All results obtained from models cargoes and NTRs used will be cross-validated and compared for functional import in cell-based assays. To interpret experimental observations and to provide fundamental physical insight, we supplement our experimental efforts with multi-component phase field modeling of LLPS. This method allows access to macroscopic time and length scales and is therefore highly compatible with the spatiotemporal regime associated with the in vitro experimentation.
