Polymers and alcohol: The story of competing depletion forces
In a standard poor solvent, polymers collapse when the solvent-monomer repulsion becomes larger than the monomer-monomer repulsion. This induces an effective attraction between monomers and results in a negative second virial coefficient between monomers. This attraction, known as depletion induced attraction, is well known from the colloidal science. However, when another poor cosolvents is added into the solution, e.g. alcohol to water, polymers can swell within intermediate mixing ratios of the poor solvents. This phenomenon is called co-solvency. A typical example is PMMA intoxicated by a water alcohol mixture. PMMA is insoluble in water as well as in pure alcohol, while it becomes better soluble in aqueous alcohol mixtures (at concentrations typical for many drinks).
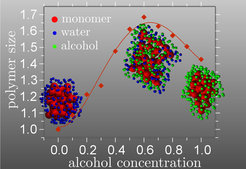
They have shown that the swelling under co-solvency condition is a second order effect, originating from the fact that solvent and cosolvent deplete both monomer solvent, cosolvent contacts and at the same time deplete each other. These competing effects reduce (or deplete) the total depletion induced force between two monomers. Furthermore, even when the polymer swells within the intermediate mixing ratios, it does not open up to its fully extended coil conformation and remains in a state where the still globally collapsed polymer consists, however, of rather large theta-blobs.
This phenomenon, which was not understood until now, provides guiding principles for tuning and designing advanced functional `smart' polymeric materials. Additionally it also provides a first molecular level picture of penetration of certain solvent mixtures into polymer containers, an often destructive phenomenon known as case II diffusion. Case II diffusion results in stress buildup and cracks in polymeric containers exposed to solvents. “While starting from the rather special case of PMMA in water alcohol mixtures, we arrived at a rather generic scheme for the behavior of polymers in poor solvent mixtures as it is relevant for many technical problems, such those related to case II diffusion,” explains Kurt Kremer, director at the Max Planck Institute for Polymer Research.
